First of all, let's get to know paraformaldehyde and formaldehyde.
What is paraformaldehyde?

- Paraformaldehyde (C2H6O2) is a white solid with a slight pungent odor. It is a polymer formed from formaldehyde.
- Paraformaldehyde is a product obtained by the polymerization of formaldehyde. At room temperature and pressure, it is a white crystalline solid compound, mostly commercially available as a white powder.
- Paraformaldehyde with longer polymer chains is used as thermoplastic material. It also participates in the synthesis of aromatic aldehydes and esters as an external CO source.

What is formaldehyde?
- Formaldehyde (chemical formula CH2O) is the simplest aldehyde in the aldehyde family. At room temperature and pressure, formaldehyde is a colorless gas with a pungent odor.
- Formaldehyde is used as a precursor in many organic synthesis processes; for example: melamine resins, phenolic resins and other resins. In addition to this, it is also used as a disinfectant.
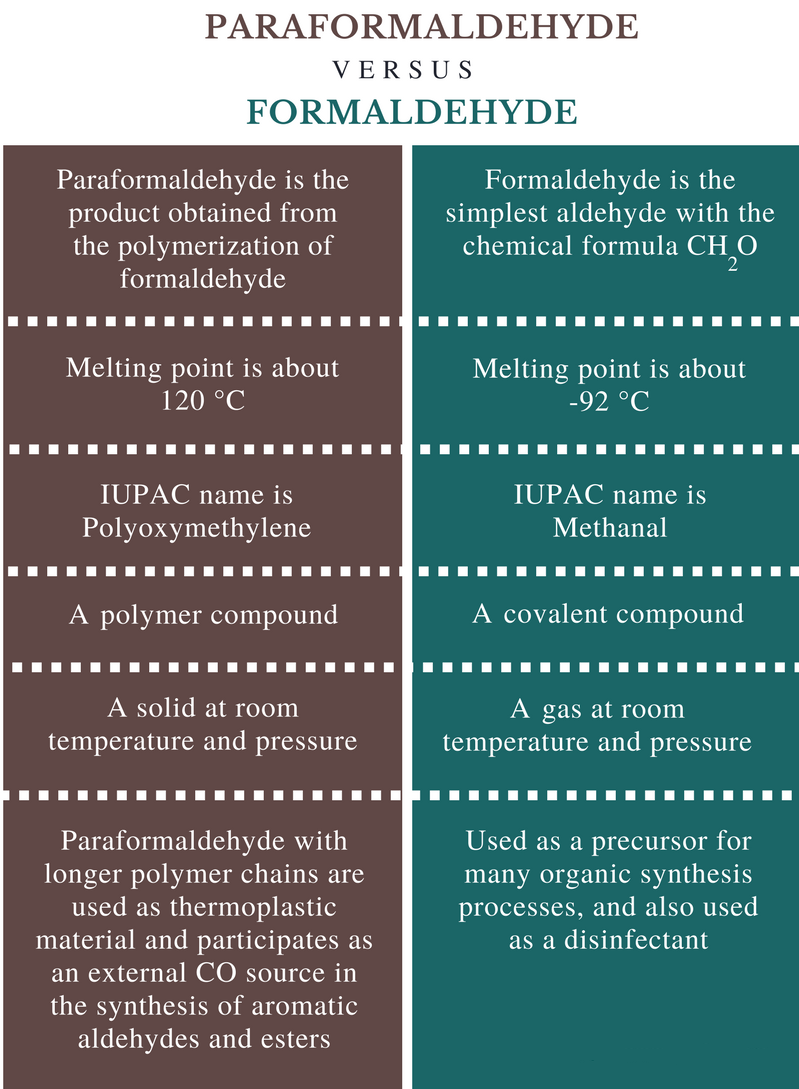
The difference between paraformaldehyde and formaldehyde
1. Chemical formula
Paraformaldehyde: The chemical formula of paraformaldehyde is C2H6O2
Formaldehyde: The chemical formula for formaldehyde is CH2O.
2. Melting point
Paraformaldehyde: The melting point of paraformaldehyde is about 120°C.
Formaldehyde: The melting point of formaldehyde is about -92°C.
3. Nature
Paraformaldehyde: Paraformaldehyde is a polymer compound.
Formaldehyde: Formaldehyde is a covalent compound.
4. Appearance
Paraformaldehyde: Paraformaldehyde is a solid at room temperature and pressure.
Formaldehyde: Formaldehyde is a gas at normal temperature and pressure.
5. Application
Paraformaldehyde: Paraformaldehyde with longer polymer chains is used as a thermoplastic material and participates as an external CO source for the synthesis of aromatic aldehydes and esters.
Formaldehyde: Formaldehyde is used as a precursor in many organic synthesis processes, as a disinfectant, etc.
6. Conclusion
Formaldehyde is a monomeric compound. Paraformaldehyde is a polymer. Paraformaldehyde is polymerized from formaldehyde. The key difference between paraformaldehyde and formaldehyde is that paraformaldehyde is a solid phase at room temperature and pressure whereas formaldehyde is a gas.


















